Do we need new Centres for Drug Control and Health Promotion?
CDC/FDA publish, analyze, and dispel safety signal with bivalent booster within 24 hours; hundreds of earlier signals ignored; Australian data show similar concerns for mRNA vaccine safety
Safety signal for stroke in older booster recipients
A few days ago, the American Centers for Disease Control and Prevention (CDC) in conjunction with the US Food and Drug Administration (FDA) issued a press release about a “Preliminary COVID-19 Vaccine Safety Signal for Persons Aged 65 Years and Older”. The agencies report that the data collected in one of their surveillance systems, the Vaccine Safety Datalink (VSD), “met the statistical criteria to prompt additional investigation into whether there was a safety concern for ischemic stroke in people ages 65 and older who received the Pfizer-BioNTech COVID-19 Vaccine, Bivalent.” The signal seems to consist of a higher stroke risk within the first three weeks after vaccination as compared to the next three weeks, i.e. weeks 4-6 after receiving the updated mRNA shots.
Intuitively, one would assume right away that a “safety signal” is just that — a signal for a possible concern that needs to be examined more closely. Just to be sure, the CDC communicators sprinkle their statements with various attenuating adjectives such as “possible”, “preliminary”, and “very unlikely … true”. Just for fun, I marked up the press release with green for positive, comforting statements praising the CDC’s extensive safety surveillance, transparency, and alternative explanations for safety signals, and red for passages referring to the actual concern at hand. About 80% of the press release is used to distract from the concern, see left half of the following figure.

The fatuitous mainstream journalists picking up the story do their part in further diminishing the bivalent booster safety concern. Within the selection of headlines captured in the above screenshot from January 14, CNN is the most truthful to the contradictory agency statements, reporting that “CDC identifies possible safety issue with Pfizer’s updated Covid-19 vaccine but says people should still get boosted”. CNBC’s story “CDC says it’s ‘very unlikely’ Pfizer booster carries stroke risk for seniors after launching review” parrots the agencies’ foregone conclusions, while Forbes makes that conclusion their own by headlining “CDC Exploring Possible But Unlikely Link Between Covid-19 Bivalent Booster And Strokes“.
Most irresponsibly, the Washington Post and New York Times are suggesting that the results of an in-depth analysis are already in: “Extensive review affirms covid booster is safe after system flagged risk” and “No Increased Stroke Risk Linked to Pfizer’s Covid Boosters, Federal Officials Say”. This claim seems to be based on background information released to the media clarifying that the safety signal from the VSD was detected in late November and, between then and now, the CDC did not find corresponding signals for stroke in the other available monitoring systems. However, the press release does not mention this timeline and is rather cautiously phrased around the other non-findings. For example, they note that the Vaccine Adverse Event Reporting System (VAERS) did not show increased reporting of stroke after the introduction of the bivalent booster product, without specifying whether the reporting was already high before the updated shots... Either way, looking into a few other drug safety surveillance systems over a 24-hour, or maybe six-week, period does not constitute an “extensive review” in my books.
Overnight, retired tech entrepreneur and founder of the Vaccine Safety Research Foundation, Steve Kirsch, has written a damning post about the regulatory agencies’ and news media’s continued recklessness. Moreover, Dr. Vinay Prasad, professor of epidemiology and biostatistics at the University of California, San Francisco, and usually a moderate critic of the COVID-19 pandemic response, doesn’t mince his words when commenting about the continued push for more doses and the earlier dismissal of safety signals that are now widely acknowledged:
This administration's vaccine policy has been horrible, and always erred on the side of pushing doses. They initially denied the safety signal of myocarditis. They delayed pulling J&J in young women. They never banned Moderna in young men. And they rubber-stamped kids vaccines with inadequate efficacy data. All the while, they made no exemption for prior infection. They pushed vaccines in a reckless way, so much so that Marion Gruber and Phil Krause resigned from FDA’s vaccine division. The worst offenders have been: Walensky, Murthy, Jha, Califf, Fauci and Marks.
Hundreds of safety signals in VAERS
Just two weeks ago, news broke that the CDC has in fact been aware of hundreds of safety signals with the COVID-19 vaccines, based on their VAERS database. Investigative journalist Zachary Stieber with The Epoch Times successfully requested access to the CDC’s internal safety monitoring data. A copy of Stieber’s original report “CDC Finds Hundreds of Safety Signals for Pfizer, Moderna COVID Vaccines” is hosted by Children’s Health Defense. It includes direct links to the released spreadsheets.
Under the Freedom of Information Act, the proportional reporting ratios (PRR) for a large number of adverse events were released, which include an increased risk of ischaemic stroke in contradiction to the CDC’s “extensive review” results reported in the first section of this post. The PRR metric is used to compare reports received for one product with a selection of reports received for other products within the same database, ensuring a degree of internal validity of the calculations. In this case, adverse events reported between December 2020 and July 2022 for the Pfizer and Moderna mRNA vaccines were compared with each other and with all vaccine injury reports received in VAERS since January 2009. The reporting data are provided for three age groups, 5-11, 12-17, and over 18 year olds, and also separated by serious and non-serious adverse events in addition to the combined tabulation.
Many of you will already have read Dr. Joshua Guetzkow’s “CDC Finally Released Its VAERS Safety Monitoring Analyses for COVID Vaccines via FOIA”, which provides thorough explanations of vaccine safety monitoring techniques and a scathing commentary on the now-published, interim results. Here, I will review a few observations of interest and then turn to a similar Australian data release for the last section of the post.

In the screenshot above, you can see the explanatory header of one of the data tables released to The Epoch Times along with the data for nine adverse event codes that contain the word “stroke”. All nine stroke conditions could have triggered safety signals as their PRRs and all-but-one chi-square values are above the CDC’s thresholds (>=2.0 and >=4.0, respectively). I am however unsure about the impact of the minimum weekly (?) counts, as the differences between the columns for July 29 and July 22 are between -1 and +1 for these codes with the exception of embolic stroke with 3 new reports. Nevertheless, the large total counts suggest that the weekly report threshold (>= 3) must have been exceeded in earlier time periods.
The following barchart shows the codes with the largest July 29 PRRs, excluding the first seven that were all related to COVID-19 itself or to the handling (e.g. cooling) of the mRNA vaccine vials. These COVID-specific adverse events may not be comparable to the non-COVID data, although those data would not only include historic vaccinations but also some concurrent, non-COVID vaccinations such as the fall 2021 flu shots, which could very well be followed by a COVID-19 infection.
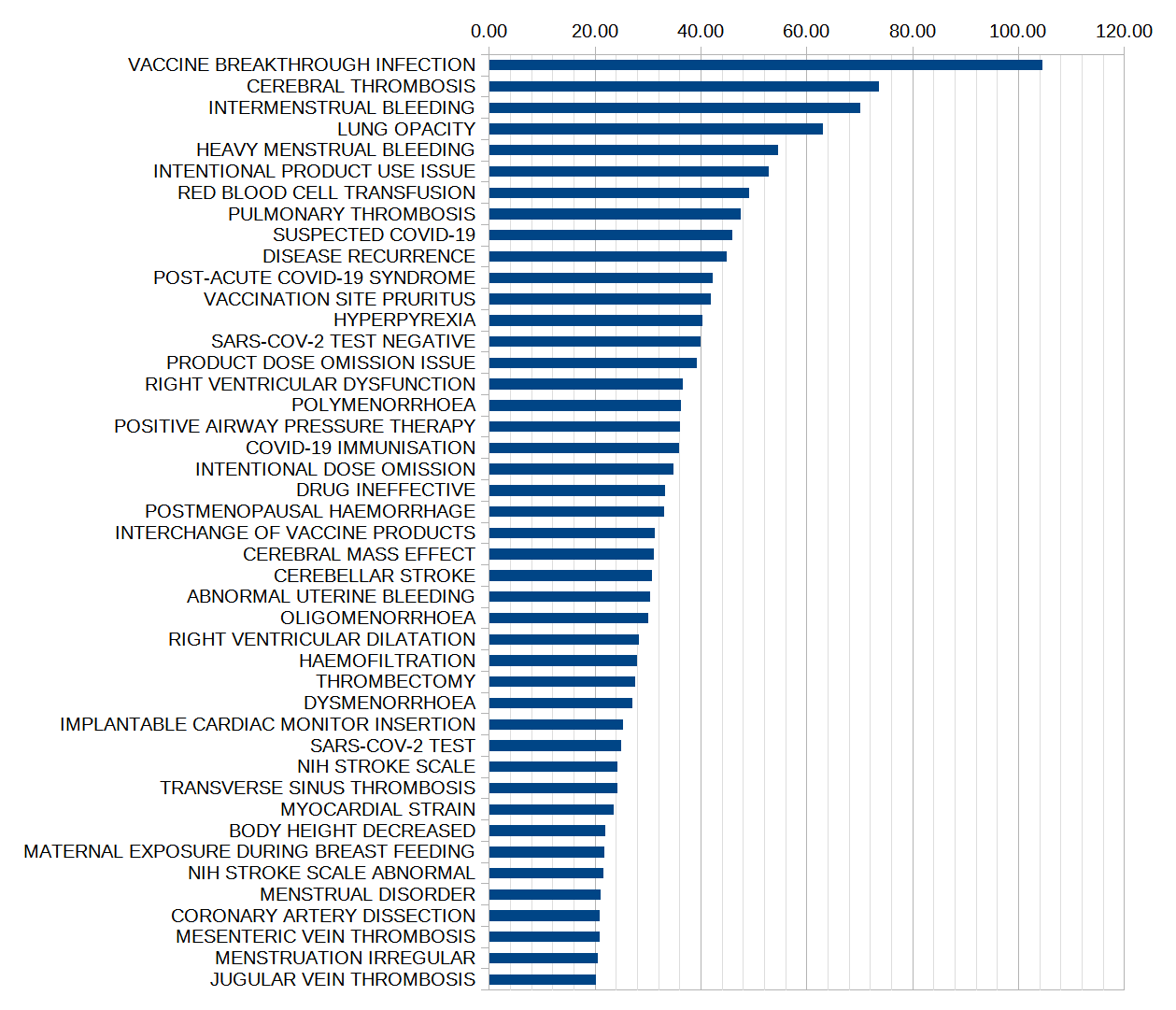
As noted by Josh Guetzkow, all of the conditions shown here and many more in the CDC data have higher safety signals than myocarditis (PRR = 3.09 in the 18+ dataset), yet myocarditis has been an adverse event of special interest since at least summer 2021, and has triggered changes to vaccination policies and vaccine information in many jurisdictions. (I have looked into myocarditis, e.g. in an early post on “The University and the Vaccine” on 21 June 2021 or in “Myocarditis under 30 is spiking - if this isn't a COVID-19 vaccine ‘safety signal’, then what is?” on 20 October 2021.)
In this context it must also be noted that the coding of adverse events results in a separation of related conditions into any number of different codes, thus potentially diluting safety signals (depending on how multiple codes might be used for a single patient). For example, the mRNA-related reports include ten codes containing “myo”, all related to heart disease and totaling 5,737 reports, as compared to “myocarditis” itself with only 2,030 reports. Again, Josh does a great job excluding the COVID-19-specific conditions and categorizing the remaining adverse events into major groups.
To replicate the CDC calculations, I created a shareable spreadsheet, which is shown below with the data for ischaemic stroke. Among over 660,000 adverse event reports made in conjunction with the mRNA shots, 488 were coded as ischaemic stroke. Since 2009, a total of 242,000+ reports were made for non-COVID vaccines, with 37 reports for ischaemic stroke among them. Thus, while there were 2.73 times as many mRNA-related reports as non-COVID reports, there were a whopping 13.19 times as many stroke reports following mRNA shots as compared to those associated with other vaccines. This results in a PRR of 4.83.

Another way of comparing the vaccine groups is also independent of the total number in each group: The 488 stroke reports represent 0.074% of all 660,643 mRNA reports, while the other 37 stroke reports represent 0.015% of all 242,091 non-COVID reports. The ratio of these two proportions, 0.074 divided by 0.15, is again 4.83 (approximately, as the values used here are rounded to two decimals). Again, I refer to Josh Guetzkow, who dispels a variety of objections regarding the use of VAERS for pharmacovigilance, including the false claim that “safety signals are due to the huge number of COVID vaccines given out”.
In my spreadsheet, I am unable to exactly replicate the chi-square value and am unsure as to why. For ischaemic stroke, my chi-square is 104.52 versus the CDC’s 103.61. The larger the chi-square statistic, the more confident we can be that two variables are not independent. The “null hypothesis” for the example is that strokes do not occur more frequently if you get an mRNA shot as compared to getting e.g. a flu shot. The high chi-square values are statistical proof that strokes occur significantly more likely with the mRNA shots. Although this safety signal does not prove a causal relationship, it demonstrates a need for further investigation using other methods, e.g. from biomedical research.
You can make a copy of the Google spreadsheet and edit the cells for other adverse events (purple) and other age groups (blue) with the data from the CDC spreadsheets.
Safety signals in Australia’s Proportionality Reporting Ratio analyses
On 29 November 2022, the Therapeutic Goods Authority (TGA) within Australia’s Department of Health disclosed a set of documents titled “Proportionality Reporting Ratio analyses for the COVID-19 vaccines to 22 October 2022” (FOI 4032). On December 12, Senator Gerrard Rennick issued a short statement titled “FOI shows injury rate significantly higher than other vaccines”. He points out the many high PRRs in these data, which suggest that several hundreds of mRNA vaccine safety signals should have been triggered in Australia.
Rennick also accuses the TGA of a lack of transparency given the limited usability of the disclosed files. The screenshot used in his post seems to be collated from different rows in FOI 4032 document 9. For example, it contains two entries for Myocarditis, which are also included in the original document but on different pages. The existence of duplicates in the adverse-event categories alone illustrates serious issues with the released TGA data (and possibly their internal database management).

My screenshot shows the first 25 rows of document 9. The column “Reaction” contains the labels of different adverse events. Two other columns contain the PRR and its “LCI”, likely the lower-confidence interval, a statistical minimum value that captures a range of uncertainty for the ratio. Each file has only one date included in the “DPAR Date” column, likely the end date for reports received over a period of a couple of months. The months for the nine documents are, in this order, March 2021, September 2021, July 2021, November 2021, January 2022, March 2022, May 2022, July 2022, and September 2022. As Senator Rennick notes, if these are based on bi-monthly reports, the data for March-May 2021 are missing.
Case counts are provided for “in period” and “total”. For example, Myocarditis is reported as follows: none (March 2021); missing (May); none (July); 94 in period/103 total (Sept); 382/494 (Nov); 445/943 (January 2022); 174/1131 and 52/145 (March, two entries); 23/133 (May, older total no longer included); 34/1058 and 3/137 (July); and 36/1066 and 12/140 (Sept). The topping off in March 2022 after a steep rise, the missing larger total in May 2022, and the subsequent steady totals are all suspicious.
I cannot make sense of the rest of the columns. Specifically, based on the magnitude and range of the values in the remaining columns, a statistical test value does not seem to be included, unlike the chi-square value in the VAERS data discussed above.

The nine PDF documents consist of an odd mix of actual data tables and images thereof, making it difficult to access the underlying data. I created a composite screenshot of the four pages of document 9 and then used an online optical character recognition (OCR) app to convert this image to an Excel spreadsheet. After numerous manual corrections of adverse event labels and missed decimal points, and re-entry of some of the misaligned case counts, I have a partially useful data table shared as a Google Sheet.
The above image shows a screenshot of the adverse events with the highest PRRs on the left and the highest total cases on the right. Similar to the VAERS data, there is a number (here 30+) of adverse events with larger safety signal (PRR) than that of the accepted vaccine side effect myocarditis. In terms of counts, myocarditis is the 9th most frequent “reaction”, but keep in mind that many other heart-related conditions also made the list of ~40 most frequent adverse events, ranging from >11,000 reports of chest pain through (myo)pericarditis and cardiac flutter. In fact, close to one half of the conditions shown above appear to be related to the cardio-vascular system.
Referring to the earlier parts of this post, ischaemic stroke, and in fact any “stroke”, is absent from the Australian September 2022 data, but stroke and related adverse events feature in the May 2022 (document 7) table, including cerebral infarction (PRR 6.26, total count 34); cerebrovascular accident (4.31, 303); intracranial haemorrhage (8.22, 21); intracranial aneurysm (3.65, 7); ischaemic stroke (5.84, 28); and transient ischaemic attack (4.11, 109). Again, these are highly concerning signals for Australians, which should have triggered investigations some 8-10 months ago. One has to wonder why all but the cerebrovascular accident category are missing from the latest spreadsheet (document 9).
All of these mRNA “vaccine” safety concerns suggest that agencies like the CDC and TGA are not doing their job, to control and prevent disease. At present, the CDC would perhaps be more aptly named Centers for Drug Culture and Pharma Propaganda. Instead, we need independent agencies that take our side by critically monitoring drug safety, reducing our dependency on the pharmaceutical industry, and promoting real health: the Centres for Drug Control and Health Promotion!





